Biodegradable and Biocompatible Polymers for Tissue Engineering Application a Review
Abstract
Polymeric biomaterials take meaning impact in the anile society. Biocompatible and biodegradable polymers take emerged during the past decades to promise extraordinary breakthroughs in a wide range of diagnostic and therapeutic medical devices. Understanding and controlling the interfacial interactions of the polymeric biomaterials with biological elements, such every bit water, ions, proteins, bacteria, fungai and cells, are essential toward their successful implementation in biomedical applications. Hither we highlight the recent developments of biocompatible and biodegradable fusion polymeric biomaterials for medical devices and provide an overview of the recent progress of the blueprint of the multi-functional biomedical polymers by controlling bio-interfacial water construction through precision polymer synthesis and supramolecular chemistry.
Introduction
In biomedical applications, there are continuous efforts to enhance methods, materials and devices. The recent evolution of novel biomaterials and their applications to biomedical problems have dramatically improved the treatment of many diseases and injuries.1, 2, iii Although a various types of materials in biomedicine have been used widely, most biomaterials lack the desired functional properties to interface with biological systems and have not been engineered for optimum performance. Therefore there is an increasing demand to develop novel materials to address such problems in biomedicine loonshit. Biocompatible and biodegradable fusion polymers are a class of new generation of biomaterials that have demonstrated great potential for medical devices, tissue engineering scaffolds, drug commitment and biomedical-healthcare sensors.
There are numerous parameters of polymeric biomaterials that tin can affect the cellular behavior in a controlled style. The underlying mechanisms for the biocompatibility of polymers at the molecular level are circuitous and have not been clearly demonstrated, although many theoretical and experimental efforts have been fabricated to understand these mechanisms.4, 5 Water and proteins interactions have been recognized as central for the biological response upon contact with polymers. We accept proposed the 'Intermediate Water' concept6, 7, viii on the basis of results on the water sorption procedure into polymeric biomaterials. The water exhibited clearly defined peaks for cold crystallization in the differential scanning calorimetry (DSC) chart, a stiff tiptop at 3400 cm−1 in a time-resolved infrared spectrum and college mobility of water in a 2H-nuclear magnetic resonance.6, 7, 8 Every bit a upshot, the biocompatibility of polymers was ascribed to the predominant population of intermediate water in the hydrated polymers. Intermediate water interacts with polymer chains in a intermediate way, that is, stronger than gratis water but weaker than tightly bound non-freezing water. We hypothesized that intermediate h2o, which prevents the proteins and blood cells from direct contacting the polymer surface on the polymer surface, has an important office in the biocompatibility of polymers.
In this focus review, we describe the recent design of biocompatible and biodegradable polymeric biomaterials for various applications in medical devices. Here we present diverse synthetic strategies for the preparation of the biomaterials, which include feature properties of the biocompatibility, biodegradability and anti-microbial activity of polymer-based biomaterials in a self-organization manner. In addition, we depict the applications of polymer-based biomaterials in tissue applied science and medical devices and provide an overview of the recent experimental progress of the screening of multi-functional biocompatible polymers based on bio-interfacial water construction.
Biocompatible polymeric biomaterials
Polymeric materials for the medical devices that may come up in contact with human blood should have capacity to resist protein adsorption and claret cell adhesion and thus triggering the organism's defense systems.one Some biocompatible polymer surfaces have been developed, and they fall into the following iii categories:ane, half dozen (a) hydrophilic surfaces, (b) surfaces with micro-stage-separated domains, and (c) biomembrane-like surfaces, including zwitterionic groups. Physicochemical properties, including wettability, surface energy, surface accuse, stiffness, topography and the presence of specific chemical functionalities, surface bound water appears to bear an instrumental office in the biological response induced by the synthetic polymers.1, 9 New-generation polymer poly(2-methoxyethyl acrylate) (PMEA) shows splendid claret compatibility and biocompatibility and has been approved for medical use by the Food and Drug Assistants.half dozen, vii, eight For example, PMEA-coated circuits and tubes exhibit significantly reduced claret cell activation when used in cardiopulmonary featherbed and catheters for primal veins of homo blood vessels. It has been maintained that PMEA's compatibility with platelets, white and red claret cells (RBCs), complement and coagulation systems has been dictated by the presence of the intermediate water.6, 7, 8
It should exist noted that the word 'biocompatibility' is used in full general as the term evaluating properties of materials that do non cause adverse consequence when the materials come up into contact with living organisms, such every bit proteins, biological cells and tissues.5 This review primarily deals with 'biocompatibility' of polymer materials against various biological elements in human claret menstruation organisation.
Principle of jail cell attachment on polymers
Cells tin can adhere in serum-containing medium even on polymers, such as polystyrene and polyethylene terephthalate, which do not possess any specific jail cell attachment ligands.ten, 11 On these polymers, serum proteins (for instance, fibrinogen and fibronectin) generally adsorb and modify their conformation to allow the cells to attach or to function equally cell zipper ligands (Figure 1a).10, 11 Poly peptide adsorption and its conformational change are thus disquisitional for cell zipper on polymers, and the regulation of protein adsorption leads to the command of cell attachment on polymers. We have suggested that intermediate water can influence protein adsorption on polymers.12, xiii Therefore the zipper behavior of the cells will be different between PMEA and conventional polymers such as polystyrene due to the difference of protein adsorption and its conformational change.
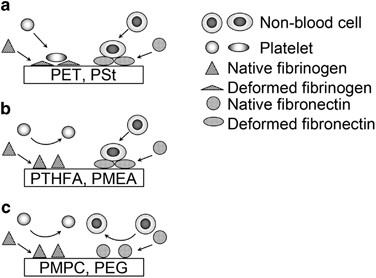
Schematic illustration of cell attachment on polymers, (a) conventional polymers (polyethylene terephthalate (PET) and polystyrene (PSt)), (b) PTFHA, PMEA and PMEA coordinating polymers. (c) PMPC and polyethylene glycol (PEG).
Different attachment of homo platelets and non-blood cells on PMEA and its analogous polymers
Cell attachment ligands are different among the cells. It has been reported that platelets require the adsorption of fibrinogen and adsorption-induced conformational change, which exposes cell attachment sites for their attachment.11 Therefore, it is necessary to prevent the adsorption and conformational change of fibrinogen on the polymers for the revenue of blood compatibility. In contrast to platelet zipper, non-blood cells require the adsorption and the conformational modify of fibronectin rather than fibrinogen for their attachment. Previously reported blood compatible polymers such as polyethylene glycol and the polymers containing 2-methacryloyloxyethyl phosphorylcholine (PMPC) take been reported to foreclose the adsorption and the conformational changes of whatsoever proteins, including both fibrinogen and fibronectin, and thus whatever types of cells cannot attach on the substrates coated with them (Figure 1c).14, 15
PMEA and its analogous polymer, poly(tetrahydrofurfuryl acrylate) (PTHFA), have been reported as blood compatible polymers.xvi, 17 These polymers suppress the adsorption and conformational change of fibrinogen to prevent platelet attachment.12, 16 Recently, we have reported that PMEA and PTHFA do non suppress the conformational change of fibronectin, and the fibronectin can expose their cell attachment sites on the polymers.13 Non-blood cells can attach on PMEA and PTHFA due to such fibronectin (Figure 1b).thirteen PMEA and PTHFA are thus newly categorized as blood compatible polymers, which permit the zipper of non-blood cells but non platelets.xiii, eighteen
Adsorption-induced conformational alter is determined past protein flexibility. The difference of conformational change between fibronectin and fibrinogen observed on PMEA and PTHFA might be due to the divergence of flexibility of these proteins. Fibronectin shapes 'chaplet on strings' and shows high flexibility.19, xx Fibronectin can modify its conformation even on the polymers that forestall conformational change of adsorbed fibrinogen. Intermediate water keeps proteins abroad from non-freezing water, which induces conformational alter of poly peptide.12, 16 It appears that necessary amounts of intermediate h2o to preclude conformational change are unlike betwixt fibronectin and fibrinogen due to the difference of flexibility. Therefore prison cell zipper can exist regulated past the regulation of intermediate water content through the regulation of poly peptide conformational change (Table i).
Recent advances in biological science and medicine require blood-contact biomedical applications, including prison cell isolation from blood and endothelial jail cell-covered artificial blood vessels and stents. Newly categorized blood-compatible polymers, such as PMEA and PTHFA, are useful for these applications. Therefore the techniques to control intermediate h2o contents will strongly progress claret-contact biomedical applications through the regulation of protein adsorption and the following jail cell attachment.
Controls on water structure at the biointerface through precision polymer synthesis
Precision control over the polymers' biocompatibility is a longstanding drawback in the arena of biocompatible polymeric materials, and the synthesis of well-defined polymers having precisely controlled molecular architecture is a powerful approach for the manipulation of polymer properties. This is particularly true in the evolution of biocompatible polymeric materials where the chief structure of polymers, for example, molecular weight, molecular weight distribution, monomer sequence distribution, stereoregularity, side-chain functionality, chain-end structures and long-chain branching, tin profoundly affect the biocompatibility of polymeric materials. To clarify the fundamental human relationship betwixt the biocompatible property of polymers and the chemical construction of polymeric biomaterials, we accept started a report to elucidate the structure-property relationships in blood-uniform polymers by means of precision polymer synthesis.
Thus far, we have been investigating the relationship between the polymer primary structures and their blood compatibility past utilizing vinyl polymers having hydrophilic functional groups. In our previous studies, we have reported that PMEA, which has a quite unproblematic chemical structure, exhibits superior blood compatibility;21, 22, 23 and PMEA possesses the unique hydration water structure, intermediate water in the hydrated state.24, 25, 26, 27, 28, 29 We accept farther investigated the blood compatibility of PMEA analogous polymers (Effigy ii) having differences in the courage construction (acrylate or methacrylate), oligo(ethylene glycol) (EG) side-chain lengths (number of units=1 to 3) and side-concatenation terminal groups (methyl or ethyl).30
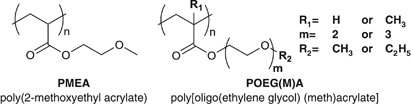
Chemical structures of poly(2-methoxyethyl acrylate) (PMEA) and PMEA coordinating polymers (POEG(M)A).
Side-chain modification
The modification with oligoEGs is a well-established methodology to tune the hydrophilicity of polymeric materials.31, 32, 33, 34, 35, 36, 37, 38, 39 Poly[oligo(ethylene glycol)(meth)acrylate]s (POEG(Thousand)As) consist of poly(meth)acrylate backbones and oligo(ethylene glycol) side-chains, consequently, the EG functionalized poly(meth)acrylate is one of the most readily accessible hydrophilic polymers. Although POEG(Thousand)As have unproblematic chemical structures and numerous inquiry studies have been conducted to appointment, in that location is nevertheless plenty of room for controlling hydrophilicity/hydrophobicity past modifying the chemical structure of side-chains. The basic way to modify the side-chain structure is past tuning the number of EG units and chain-cease last group. Hydrophilicity of the polymer increases with the number of EG units, as the polymers have longer side-chains, the polymers become soluble in water and typically show lower critical solution temperature (LCST) in aqueous solutions.33 The number of carbon atoms in terminal alkoxy group besides affects the water solubility and some of the polymers having longer alkyl terminal group testify LCST beneath 37 °C.xl The DSC measurement revealed that intermediate water content was increased by tuning the chemic construction of polymer to be more hydrophilic (much EG units with less terminal carbons), and a decrement trend was observed in the number of adhered platelets with increasing the intermediate water content.
Zwitterionic polymers are known as the promising biocompatible materials for medical devices.40 For case, poly(2-methacryloyloxyethyl phosphorylcholine) (PPBMA: poly(phosphobetaine methacrylate), mostly known as PMPC)) is a biomimetic fabric containing phosphorylcholine group for resisting nonspecific poly peptide adsorption and platelet adhesion.41, 42 Recently, constructed polymers containing zwitterionic structures similar to PPBMA, such equally poly{[2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide} (poly(sulfobetaine methacrylate)),43, 44 and poly(1-carboxy-N,North-dimethyl-N-(2'-methacryloyloxyethyl)methanaminium) (poly(carboxybetaine methacrylate)),45, 46, 47 bearing sulfo- and carboxy- betaine group, respectively, are also reported as blood-compatible polymers, which show good plasma protein-fouling resistance. Almost recently, poly(serine methacrylate) was reported as a new family of a zwitterionic polymer having an amino acrid, L-serine, every bit the side-chain group.48 (Figure 3)
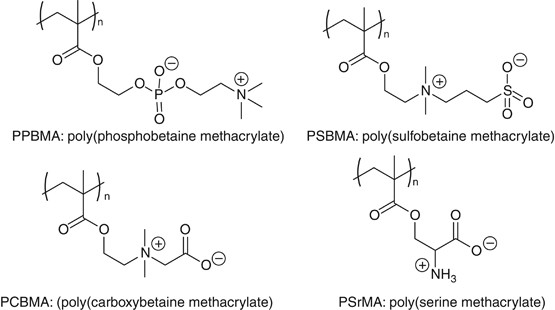
Chemical structures of zwitterionic polymers (phospho-betaine, sulfo-betaine and calboxy-betaine), which possess the intermediate water in their hydrated state.
Backbone modification
Irresolute the polymer backbone construction is also an effective approach to tune the polymer properties.49, 50 Poly[oligo(ethylene glycol) vinyl ether] is an analog of POEG(M)A. The structural difference betwixt the ii polymers is simply in the side-concatenation linkage that the former OEG side-chains were connected to polymer courage through ether bonds instead of ester connections (Figure 4). However, the difference engenders big differences in the molecular mobility and the hydrophilicity of the polymers. For instance, most of poly(vinyl ether)due south having OEG side-chains show quite depression glass transition temperature (T g<−60 °C) and are soluble in water or showroom an LCST in aqueous media. Some of the poly(vinyl ether)s (for example, poly(2-ethoxyethyl vinyl ether), LCST=21 °C) are insoluble at body temperature, and the human platelets adhesion test could be performed at 37 °C. Accordingly, we take analyzed the hydration water structure in POEG(M)A and their poly(vinyl ether) analogs by DSC, and the poly(vinyl ether)s showed a cold crystallization of h2o at around −forty °C and exhibited low platelet adhesion also as the example of POEG(G)A.51
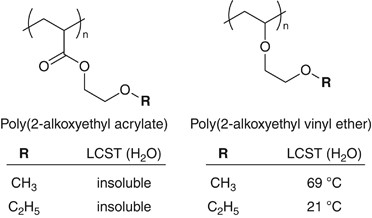
Poly(2-alkoxyethyl acrylate)due south and their poly(vinyl ether) analogs. Poly(two-ethoxyethyl vinyl ether) (R=C2H5) exhibits LCST at 21 °C.
Modification on side-chain branch spacing
As mentioned above, the structural control over the macromolecular chemical structure is an constructive approach to modify/control the hydration water structure and the blood compatibility of polymeric materials. There remains ample telescopic for further modification in the chemical structure of polymers, for example, tacticity, side-concatenation linkage and side-chain branch placement. The structural control over the side-chain placement along the polymer backbone is one of the most challenging topics in vinyl polymer synthesis. Fortunately, an constructive pathway to achieve the model sequence-regulated vinyl polymers was reported, and the methodology utilizing the regio- and stereo-selective band-opening metathesis polymerization (ROMP) of allyl-substituted cycloalkenamers52 opened a new window to precisely command the side-concatenation branch placement.53, 54, 55, 56, 57, 58 Based on the works, we have started a study to elucidate the structure–property relationships in biocompatible polymeric materials past means of precision polymers synthesized through regio- and stereo-selective ROMP (Figure 5).
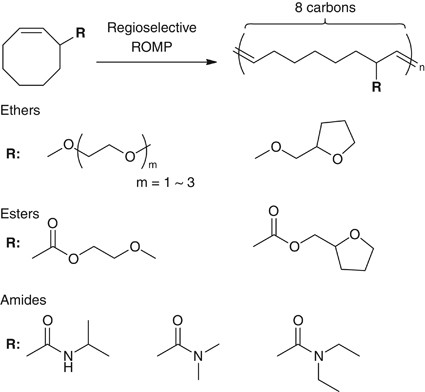
Synthesis of polymers having precisely placed side-chain branches by regio- and stereo-selective ROMP of allyl-substituted cis-cyclooctenes.
The single commutation of a functional group at the allyl-position of cis-cyclooctene (COE) allows to achieve the regioregular polymers by means of ROMP with the 2nd-generation Grubbs goad ( G2 ).59 Thus we have synthesized COEs having hydrophilic functional groups at allyl-position,60 for example, polymerized the COEs with G2 in CHCl3. ROMP of the allyl-substituted COEs proceeded in a regio- and stereo-selective fashion to afford polymers exhibiting remarkably high caput-to-tail regioregularity and high trans- stereo-regularity as nosotros previously reported. Effigy 6 shows olefinic region of aneH nuclear magnetic resonance and 1H-1H correlated spectra of 3-methoxy-substituted COE. The coupling constant for the ii olefinic signals is J ab=15.5 Hz, indicating that the double bond has trans- configuration. The dd and dt multiplicities for Ha and Hb, respectively, and the correlation between Ha and Hb reveal the most-perfect trans- caput-to-tail regularity.
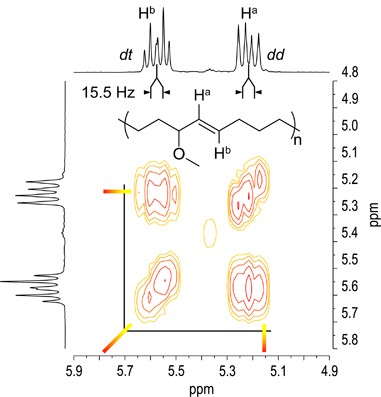
Olefinic region of 1H NMR spectra and iH-1H correlated spectra of poly(three-methoxy-one-cyclooctene).
Polymers having precisely placed branches on every eighth backbone carbons were obtained upon hydrogenation. Water contact angle measurement confirmed the presence of hydrophilic surface for all polymers. The h2o structure in hydrated polymers was determined by DSC, common cold crystallization of water and/or low melting of ice in hydrated polymers were observed on heating process. Common cold crystallization of water is the clear bear witness for the presence of intermediate h2o, and the content was able to be varied by changing the polymer structure. A homo platelet adhesion test was employed to assess the blood compatibility of regioselective ROMP-produced polymers. The number of adhered platelets was also varied past changing the polymer structure, and we found out that the number was suppressed past introducing the longer EG side-chains. The platelets adhesion number was decreased with increasing the content of intermediate water regardless of the polymer structure. This result suggests that our hypothesis could exist true that the presence of intermediate h2o is the key to provide the polymer materials with antithrombotic grapheme, and the claret compatibility of polymers should be controlled by tuning the water construction at the bio-interface through precision polymer synthesis.60
Biodegradable constructed polymers used/studied in medical applications
Some biomedical devices, peculiarly for temporary use or dispensable purpose, such equally surgical suture, bone-fixation materials and drug-eluting stents comprise biodegradable synthetic polymers, including polylactides, polyglycolide, poly(ɛ-caprolactone), poly(trimethylene carbonate) (PTMC) and poly(p-dioxanone), every bit shown in Figure 7.61, 62 These polymers are degraded by hydrolysis with/without enzyme and absorbed in the body through metabolic pathway, although the duration of these existing biodegradable polymers in the body varies.63 They have drawn not bad attention as alternatives to biopolymers such as peptides, nucleic acids and polysaccharides that cost high to produce and purify and potentially possess the gamble of antigenicity and infection.
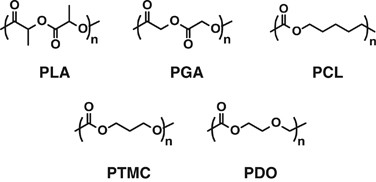
Biodegradable polymers used/studied in medical applications.
Such implantable medical devices need to be compatible with host cells to reduce adverse effects. As it has been confirmed that the aforementioned polymers exhibit minimal or acceptable cytotoxicity,64 almost of those polymers are approved for medical application. Few reports have ever described the human relationship betwixt the biocompatibility and structural features of those polymers. In the instance of PMEA, the ester and ether groups on the side-chains contribute to the hydration and generation of intermediate water.29 The hydration generally occurs through hydrogen bonding betwixt polar moieties in the polymer and water molecules. This concept may be extended to the aforementioned polymers comprising ester or carbonate linkage and alkyl- or alkyloxy-bondage of varying lengths. The detailed study for hydration and intermediate water in those polymers is now in progress by our grouping. Next examples also imply that the intermediate water concept should exist employed to explain the observed biocompatibility.
Biodegradable antimicrobial polymers with depression hemolytic belongings
In recent years, a PTMC analog bearing a side grouping of quaternary ammonium salt take demonstrated strong antimicrobial activities but showed minimal hemolytic properties (Figure 8).65, 66 In contrast, almost of the cationic polymers are well known to collaborate with negatively charged bacterial cell membranes, subsequently inducing the membrane disruption.67 As the cationic polymers physically destroy cells, drug resistance is hard to develop differently from the use of conventional antibodies. Even so, this electrostatic interaction often influences mammalian cells resulting in cytotoxicity, which is a serious effect to be solved in developing antimicrobial polymers with positive charges. The first antimicrobial polycarbonate reported in 2011 shows efficient antimicrobial action but displays no hemolytic property.65 This polymer has amphiphilic triblock nature to form nano-sized micelles by conjugating hydrophobic PTMC equally peripheral blocks (Effigy 8a). Accumulation of positive charges on micelle surface might contribute to differentiating bacteria and mammalian cells. In like speculation proposed by Kuroda and colleagues, localization of charges and segregation of the hydrophobic part past micellization suppress the interaction with mammalian cell membrane with less negative charges than those of bacterial prison cell membrane.68, 69 Considering that the other PTMC analogs bearing different side-chains have too exhibited piffling cytotoxicity;70, 71, 72 even so, this low hemolytic property may also be supported by the contribution of hydration involving the carbonate linkages in the master chain. In particular, equally both RBCs and platelets are blood cells, the inactive behavior of the polymer to RBCs is likely to occur in a similar way that PMEA shows excellent compatibility to platelets.16 IBM propounds to call a series of these antimicrobial biodegradable polycarbonates 'Ninja Polymer' describing the role to work behind the scenes and eventually disappear.
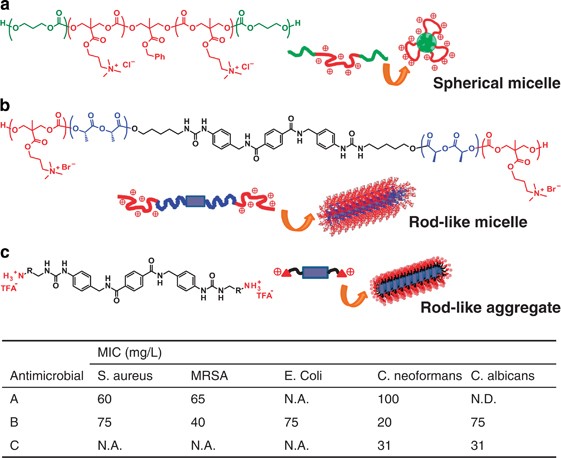
Synthetic biodegradable antimicrobials in unlike agile forms. (a) spherical micelles (critical micelle concentration (CMC)=28 μg ml−1), (b) rod-like micelles (CMC=25 μg ml−1), (c) rod-like aggregate (CMC=half dozen μg ml−1).
Supramolecularly bolstered antimicrobial activity and blood compatibility
Multiple activities against several types of leaner present another claiming for the design of antimicrobial materials. The beginning antimicrobial polycarbonate described above shows the efficacy just against Gram-positive bacteria and their drug-resistant strains such as Bacillus subtilis and (methicillin-resistant) Staphylococcus aureus, respectively.65 Because Gram-negative bacteria and fungi are not as negatively charged equally Gram-positive bacteria are,73 other artifices should exist integrated into the macromolecular architecture. Lipophilicity and hydrophobicity are more often than not required for valid antimicrobial activity against Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa, attributable to affinity to superficial lipopolysaccharide on the prison cell wall. Nonetheless, increased hydrophobicity of the cationic polymers often develops hemolytic property. Fukushima et al. 66 introduced a rigid hydrogen bonding motif in the middle of a center hydrophobic segment of a triblock copolymer equanimous of poly(50-lactide) (PLLA) and the cationic polycarbonate, forming gristly micelles by orientation of self-associates (Figure 8b). Interestingly, this polymer shows antimicrobial action against a broad range of bacteria covering Gram-positive/negative bacteria and fungi but induced no hemolysis. In all cases, minimum inhibitory concentration of this polymer was higher than critical micelle concentration, supporting that the polymer serves equally aggregates. Information technology turns out that the fibrous shape is somewhat responsible for the improved antimicrobial activity. Later, Fukushima et al. 74 accept as well reported supramolecular antifungals where the same rigid hydrogen bond motif is used directly to attach low molecular cationic main ammonium at the both ends instead of the cationic polycarbonate (Figure 8c). The antifungal becomes active against fungi such every bit Candida albicans and Cryptococcus neoformans only in the form of nanofiber that indicates drinking glass transition at 120 °C like to molecular drinking glass.75
In these higher up 2 cases, the hemolytic activity of the polymer also remained minimal. The cationic moiety plainly affects bacterial cell membranes, but the interaction with RBCs is mitigated even though the associates form varies. At the latter case, especially, the interaction of molecules with cells, such as cytotoxicity, antimicrobial activeness and biocompatibility, is managed by cooperation of primary structure of the peripheral functional groups to tune the chemical functions and higher-guild structure forming specific shape to restrict or expand the chemical function as biological system generally adopts. The cationic moiety usually forms hydration layer, including strongly oriented h2o molecules that are categorized as non-freezing water, often causing adverse effects. If the intermediate water is responsible for the low hemolytic property of the cationic fibrous assemblies, the following hypothesis would be supposed: By assembling to such fibrous class, the surface hydration layer is disorganized with electrostatic repulsion of condensed cationic groups, which may trigger forming intermediate water from non-freezing h2o. In fact, a similar insight has been reported for generation of intermediate water by disorganization of hydration layer of non-freezing water in a copolymer of poly[Due north-methyl-N-(4-vinylphenethyl)ethylenediamine] with a small amount of additional poly(ii-hydroxyethyl methacrylate).76
Control over the geometry of polymeric aggregates oftentimes entails non-covalent interactions, including hydrogen bond, π-π stacking, charge transfer complex and ion circuitous. These interactions also differentiate nano-rheology and dynamics of the aggregates. According to Stupp and colleagues, strength of the interaction at the internal domain of cationic supramolecular aggregates affects aggregating on and disaggregation of mammalian cell membrane.77 The aggregates with strong 'internal bond strength' interact with the cell membrane resulting in membrane disruption, while those with weak internal bond forcefulness remain dynamic nature to release unimers upon approaching cells leading to no damage of the cells. It turns out that the choice of types and management of bond (covalent vs non-covalent) significantly involves regulation of biocompatibility and cytotoxicity. In consequence, design of high-performance biomaterials in future should actively utilise supramolecular chemistry in terms of geometry control, subsequent development of secondary function and dynamic behavior of the material besides primary chemic functions.
The enquiry on clarification of relationship between biocompatibility of these polymer systems and water construction is ongoing.78 The intermediate water was only found in hydrated biopolymers (proteins, polysaccharides and nucleic acid; Deoxyribonucleic acid and RNA) and hydrated biocompatible synthetic polymers simply not in hydrated non-biocompatible synthetic polymers.79, 80, 81, 82 Therefore we propose intermediate water concept for directional design of functional polymeric biomaterials, but it is needed for the quantitative and precise clarification of biocompatibility driven by novel interface-sensitive approaches, such as spectroscopic (including sum-frequency generation and dielectric spectroscopy), 10-ray and neutron scattering, and force curve measurements combined with computer simulations under the physiological condition.83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94
Conclusion
Surfaces made of biocompatible and biodegradable polymers profoundly influence cell behavior at all hierarchical levels. The interaction of polymers with cells is managed by cooperation of primary structure including courage and functional groups at the side-chain to tune the chemical functions and higher-guild construction forming specific shape to restrict or aggrandize the chemical function equally biological organization mostly adopts. Using principles of intermediate water, which is mutual in hydrated biopolymers and in only biocompatible constructed polymers, the synthetic and supramolecular methodology to create novel biocompatible polymers moves toward a more loftier-throughput way. Such well-defined polymeric biomaterials could find application in the historic period of personalized medicine.
References
-
Severian, D . Polymeric Biomaterials, (Mercel Dekker, New York, NY, USA, 2002).
-
Karagkiozaki, V . & Logothetidis, S . Horizons in Clinical Nanomedicine, (Pan Stanford Publishing Pte. Ltd., Singapore, Singapore, 2014).
-
Bouten, C. 5. C ., Dankers, P. Y. West ., Driessen-Mol, A ., Pedron, S ., Brizard, A. M. A . & Baaijens, F. P. T . Substrates for cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 63, 221–241 (2011).
-
Ratner, B. D . Blood compatibility-a prespective. J. Biomater. Sci. Polym. Ed. 11, 1107–1119 (2000).
-
Tsuruta, T . On the role of water molecules in the interface between biological systems and polymers. J. Biomater. Sci. Polym. Ed. 21, 1831–1848 (2010).
-
Tanaka, K ., Hayashi, T . & Morita, S . The roles of water molecule at the biointerface of medical polymers. Polym. J. 45, 701–710 (2013).
-
Javakhishvili, I ., Tanaka, M ., Ogura, K ., Jankova, 1000 . & Hvilsted, S . Synthesis of graft copolymers based on poly(ii-methoxyethyl acrylate) and investigation of the associated water construction. Macromol. Rapid Commun. 33, 319–325 (2012).
-
Miwa, Y ., Ishida, H ., Saitô, H ., Tanaka, M . & Mochizuki, A . Network structures and dynamics of dry and bloated poly(acrylate)s.Label of high- and low-frequency motions every bit revealed past suppressed or recovered intensities (SRI) assay of xiiiC NMR. Polymer 50, 6091–6099 (2009).
-
Nell, A. Eastward ., Mädler, L ., Velegol, D ., Xia, T ., Hoek, Due east. M. V ., Somasundaran, P ., Klaessig, F ., Castranova, Five . & Thompson, M . Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. eight, 543–557 (2009).
-
Grinnell, F . & Feld, G. Thousand . Fibronectin adsorption on hydrophilic and hydrophobic surfaces detected by antibody bounden and analyzed during prison cell adhesion in serum-containing medium. J. Biol. Chem. 257, 4888–4893 (1982).
-
Sivaraman, B . & Latour, R. A . The relationship between platelet adhesion on surfaces and the structure versus the corporeality of adsorbed fibrinogen. Biomaterials 31, 832–839 (2010).
-
Tanaka, M ., Mochizuki, A ., Shiroya, T ., Motomura, T ., Shimura, M ., Onishi, M . & Okahata, Y . Study on kinetics of earlystage protein adsorption on poly(ii-methoxethylacrylate) (PMEA) surface. Coll. Surf. A Physicochem. Eng. Aspects 203, 195–204 (2002).
-
Hoshiba, T ., Nikaido, M . & Tanaka, G . Characterization of the attachment mechanisms of tissue-derived cell lines to claret-compatible polymers. Adv. Healthcare Mater. three, 775–784 (2014).
-
Zhang, Z ., Zhang, G ., Chen, S ., Horbett, T. A ., Ratner, B. D . & Jiang, Southward . Blood compatibility of surfaces with superlow poly peptide adsorption. Biomaterials 29, 4285–4291 (2008).
-
Ishihara, Yard ., Aragaki, R ., Ueda, T ., Watanabe, A . & Nakabayashi, N . Reduced thrombogenicity of polymers having phospholipid polar groups. J. Biomed. Mater. Res. 24, 1069–1077 (1990).
-
Tanaka, M ., Motomura, T ., Kawada, M ., Anzai, T ., Kasori, Y ., Shiroya, T ., Shimura, M ., Onishi, M . & Mochizuki, A . Blood uniform aspects of poly (2-methoxyethylacrylate) (PMEA)- relationship betwixt protein adsorption and platelet adhesion on PMEA surface. Biomaterials 21, 1471–1481 (2000).
-
Mochizuki, A ., Hatakeyama, T ., Tomono, Y . & Tanaka, M . Water structure and blood compatibility of poly (tetrahydrofurfuryl acrylate). J. Biomater. Sci. Polym. Ed. twenty, 591–603 (2009).
-
Kitakami, E ., Aoki, M ., Sato, C ., Ishihata, H . & Tanaka, M . Adhesion and proliferation of human periodontal ligament cells on poly(2-methoxyethyl acrylate). Biomed. Res. Int. 2014, 102648 (2014).
-
Erickson, H. A ., Carrell, N . & McDonagh, J . Fibronectin molecule visualized in electron microscopy: a long, thin, flexible strand. J. Jail cell Biol. 91, 673–678 (1981).
-
Leahy, D. J ., Aukhil, I . & Erickson, H. P . two.0 Å Crystal construction of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell 84, 155–164 (1996).
-
Tanaka, Thousand . & Mochizuki, A . Clarification of the claret compatibility machinery past decision-making the water structure at the blood-poly(meth)acrylate interface. J. Biomater. Sci. Polym. Ed. 21, 1849–1863 (2010).
-
Tanaka, K ., Mochizuki, A ., Ishii, N ., Motomura, T . & Hatakeyama, T . Report on blood compatibility of poly(two-methoxyethylacrylate). Relationship betwixt water structure and platelet compatibility in poly(2-methoxyethylacrylate-co-2- hydroxyethylmethacrylate). Biomacromolecules 3, 36–41 (2002).
-
Tanaka, 1000 ., Mochizuki, T ., Motomura, T ., Shimura, K ., Onishi, M . & Okahata, Y . In Situ studies on protein adsorption onto a poly(two-methoxyethylacrylate) surface past a quartz crystal microbalance. Coll. Surf. A Physicochem. Eng. Aspects 193, 145–152 (2001).
-
Tanaka, M ., Motomura, T ., Ishii, Northward ., Shimura, G ., Onishi, G ., Mochizuki, A . & Hatakeyama, T . Cold crystallization of water in hydrated poly(two-methoxyethyl acrylate) (PMEA). Polym. Int. 49, 1709–1713 (2000).
-
Tanaka, M . & Mochizuki, A . Effect of water construction on blood compatibility, thermal analysis of water in poly(meth)acrylate. J. Biomed. Mater. Res. 68A, 684–695 (2004).
-
Miwa, Y ., Ishida, H ., Tanaka, Grand . & Mochizuki, A . H-2-NMR and C-13-NMR study of the hydration behavior of poly(ii-methoxyethyl acrylate), poly(2-hydroxyethyl methacrylate) and poly(tetrahydrofurfuryl acrylate) in relation to their blood compatibility every bit biomaterials. J. Biomater. Sci. Polym. Ed. 21, 1911–1924 (2010).
-
Miwa, Y ., Tanaka, M . & Mochizuki, A . Water structure and polymer dynamics in hydrated claret compatible polymers. Kobunshi Ronbunshu 68, 133–146 (2011).
-
Hayashi, T ., Tanaka, M ., Yamamoto, Due south ., Shimomura, Grand . & Hara, M . Directly ascertainment of interaction between proteins and claret-compatible polymer surfaces. Biointerphases two, 119–125 (2007).
-
Morita, S ., Tanaka, K . & Ozaki, Y . Fourth dimension-resolved in situ ATR-IR observations of the process of sorption of water into a poly(2-methoxyethyl acrylate) picture show. Langmuir 23, 3750–3761 (2007).
-
Tanaka, M . & Ido, N . Novel biocompatible and temperature responsive polymers. Patent application JP2011, 4853905.
-
Knop, K ., Hoogenboom, R ., Fischer, D . & Schubert, U. S . Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 49, 6288–6308 (2010).
-
Veronese, F. Yard . & Pasut, K . PEGylation, successful approach to drug delivery. Drug Discov. Today 10, 1451–1458 (2005).
-
Lutz, J.-F . Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. A Polym. Chem 46, 3459–3470 (2008).
-
Han, S ., Hagiwara, M . & Ishizone, T . Synthesis of thermally sensitive h2o-soluble polymethacrylates by living anionic polymerizations of oligo(ethylene glycol) methyl ether methacrylates. Macromolecules 36, 8312–8319 (2003).
-
Kitano, H ., Hirabayashi, T ., Gemmei-Ide, M . & Kyogoku, Grand . Consequence of macrocycles on the temperature-responsiveness of poly (methoxy diethylene glycol methacrylate)-graft-PEG. Macromol. Chem. Phys. 205, 1651–1659 (2004).
-
Zhao, B ., Li, D. J ., Hua, F. J . & Green, D. R . Synthesis of thermosensitive water-soluble polystyrenics with pendant methoxyoligo(ethylene glycol) groups by nitroxide-mediated radical polymerization. Macromolecules 38, 9509–9517 (2005).
-
Neugebauer, D . Graft copolymers with poly(ethylene oxide) segments. Polym. Int. 56, 1469–1498 (2007).
-
Ishizone, T ., Seki, A ., Hagiwara, M ., Han, S ., Yokoyama, H ., Oyane, A ., Deffieux, A . & Carlotti, Southward . Anionic polymerizations of oligo(ethylene glycol) alkyl ether methacrylates: Effect of side chain length and ω-alkyl group of side concatenation on cloud point in water. Macromolecules 41, 2963–2967 (2008).
-
Yamanaka, J ., Kayasuga, T ., Ito, M ., Yokoyama, H . & Ishizone, T . Synthesis of water-soluble poly[oligo(ethylene glycol) methacrylate]s by living anionic polymerization of oligo(ethylene glycol) vinyl ether methacrylates. Polym. Chem. 2, 1837–1848 (2011).
-
Tarannum, N . & Singh, M . Advances in synthesis and applications of sulfo and carbo analogues of polybetaines: a review. Rev. Adv. Sci. Eng. ii, 90–111 (2013).
-
Ishihara, K ., Nomura, H ., Mihara, T ., Kurita, K ., Iwasaki, Y . & Nakabayashi, N . Why practise phospholipid polymers reduce poly peptide adsorption? J. Biomed. Mater. Res. 39, 323–330 (1998).
-
Ishihara, K ., Hanyuda, H . & Nakabayashi, North . Synthesis of phospholipid polymers having a urethane bond in the side chain as coating fabric on segmented polyurethane and their platelet adhesion-resistant properties. Biomaterials 16, 873–879 (1995).
-
Jun, Z ., Youling, Y ., Kehua, W ., Jian, S . & Sicong, L . Surface modification of segmented poly(ether urethane) by grafting sulfo ammonium zwitterionic monomer to improve hemocompatibilities. Colloids Surf. B 28, ane–9 (2003).
-
Lee, W.-F . & Tsai, C.-C . Synthesis and solubility of the poly(sulfobetaine)s and the corresponding cationic polymers: one. Synthesis and label of sulfobetaines and the corresponding cationic monomers by nuclear magnetic resonance spectra. Polymer 35, 2210–2217 (1994).
-
Kitano, H ., Tada, Southward ., Mori, T ., Takaha, K ., Gemmei-Ide, M ., Tanaka, M ., Fukuda, 1000 . & Yokoyama, Y . Correlation between the construction of water in the vicinity of carboxybetaine polymers and their blood-compatibility. Langmuir 21, 11932–11940 (2005).
-
Zhang, Z ., Chen, S . & Jiang, South . Dual-functional biomimetic materials: nonfouling poly(carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules 7, 3311–3315 (2006).
-
Yuan, Y ., Zang, 10 ., Ai, F ., Zhou, J ., Shen, J . & Lin, Southward . Grafting sulfobetaine monomer onto silicone surface to improve haemocompatibility. Polym. Int. 53, 121–126 (2004).
-
Liu, Q ., Singh, A . & Liu, L . Amino acid-based zwitterionic poly(serine methacrylate) every bit an antifouling material. Biomacromolecules fourteen, 226–231 (2013).
-
Fernández-García, Grand ., Cuervo-Rodriguez, R . & Madruga, East. Fifty . Glass transition temperature of methyl methacrylate–ethyl α-benzoyloxymethylacrylate copolymers. Polym. Int. 49, 377–381 (2000).
-
Cao, C . & Lin, Y . Correlation betwixt the glass transition temperatures and repeating unit structure for loftier molecular weight polymers. J. Chem. Inf. Comput. Sci. 43, 643–650 (2003).
-
Tanaka, K . Blood uniform poly(vinyl ether)s. Patent awarding pending JP2014-047347.
-
Kobayashi, S ., Pitet, L. One thousand . & Hillmyer, G. A . Regio- and stereoselective ring-opening metathesis polymerization of iii-substituted cyclooctenes. J. Am. Chem. Soc. 133, 5794–5797 (2011).
-
Jeong, H ., Kozera, D. J ., Schrock, R. R ., Smith, S. J ., Zhang, J ., Ren, N . & Hillmyer, M. A . Z-Selective ring-opening metathesis polymerization of 3-substituted cyclooctenes by monoaryloxide pyrrolide imido alkylidene (MAP) catalysts of molybdenum and tungsten. Organometallics 32, 4843–4850 (2013).
-
Martinez, H ., Miro, P ., Charbonneau, P ., Hillmyer, Chiliad. A . & Cramer, C. J . Selectivity in ring-opening metathesis polymerization of Z-cyclooctenes catalyzed by a second-generation Grubbs catalyst. ACS Catal 2, 2547–2556 (2012).
-
Martinez, H ., Ren, N ., Matta, M. E . & Hillmyer, Yard. A . Band-opening metathesis polymerization of 8-membered cyclic olefins. Polym. Chem. 5, 3507–3532 (2014).
-
Pitet, L. M ., Zhang, J . & Hillmyer, M. A . Sequential ROMP of cyclooctenes every bit a route to linear polyethylene block copolymers. Dalton Trans. 42, 9079–9088 (2013).
-
Zhang, J ., Matta, K. East . & Hillmyer, M. A . Synthesis of sequence-specific vinyl copolymers past regioselective ROMP of multiply substituted cyclooctenes. ACS Macro Lett. 1, 1383–1387 (2012).
-
Zhang, J ., Matta, Grand. E ., Martinez, H . & Hillmyer, Grand. A . Precision vinyl acetate/ethylene (VAE) copolymers by ROMP of acetoxy-substituted cyclic alkenes. Macromolecules 46, 2535–2543 (2013).
-
Martinez, H ., Zhang, J ., Kobayashi, S ., Xu, Y ., Pitet, 50. M ., Matta, Yard. Due east . & Hillmyer, Thou. A . Functionalized regio-regular linear polyethylenes from the ROMP of 3-substituted cyclooctenes. Appl. Pet. Res. (doi: ten.1007/s13203-014-0048-z).
-
Tanaka, One thousand ., Kobayashi, S ., Fukuda, K . & Herai, Grand . Biocompatible polymers having precisely placed side-chain branches. Patent application pending JP2014, 105221.
-
Nair, L. S . & Laurencin, C. T . Biodegradable polymers equally biomaterials. Prog. Polym. Sci. 32, 762–798 (2007).
-
Tian, H ., Tang, Z ., Zhuang, X ., Chen, X . & Jing, Ten . Biodegradable synthetic polymers: preparation, functionalization and biomedical awarding. Prog. Polym. Sci. 37, 237–280 (2012).
-
van der Zee, 1000 . in: Handbook of Biodegradable Polymers: Isolation, Synthesis, Characterization and Applications (eds Lendlein A. & Sisson A.) 263–281 (Wiley-VCH, Weinheim, Germany, 2011).
-
Tzoneva, R ., Seifert, B ., Behl, M . & Lendlein, A . Rubberband multiblock copolymers for vascular regeneration: protein adsorption and hemocompatibility. Clin. Hemorheol. Micro 52, 337–348 (2012).
-
Nederberg, F ., Zhang, Y ., Tan, J. P. G ., Xu, Thou ., Wang, H ., Yang, C ., Gao, Southward ., Guo, X. D ., Fukushima, K ., Li, Fifty ., Hedrick, J. L . & Yang, Y. Y . Biodegradable nanostructures with selective lysis of microbial membranes. Nat. Chem. iii, 409–412 (2011).
-
Fukushima, 1000 ., Tan, J. P. G ., Korevaar, P. A ., Yang, Y. Y ., Pitera, J ., Nelson, A ., Maune, H ., Coady, D. J ., Frommer, J. E ., Engler, A. C ., Huang, Y ., Xu, One thousand ., Ji, Z ., Qiao, Y ., Fan, W ., Li, 50 ., Wiradharma, N ., Meijer, E. W . & Hedrick, J. Fifty . Broad-spectrum antimicrobial supramolecular assemblies with distinctive size and shape. ACS Nano 6, 9191–9199 (2012).
-
Wimley, W. C . Describing the mechanism of antimicrobial peptide activity with the interfacial activity model. ACS Chem. Biol. five, 905–917 (2010).
-
Oda, Y ., Kanaoka, S ., Sato, T ., Aoshima, S . & Kuroda, K . Block versus random amphiphilic copolymers as antibacterial agents. Biomacromolecules 12, 3581–3591 (2011).
-
Palermo, E. F . & Kuroda, K . Structural determinants of antimicrobial activity in polymers which mimic host defence peptides. Appl. Microbiol. Biotechnol. 87, 1605–1615 (2010).
-
Attia, A. B. E ., Yang, C ., Tan, J. P. K ., Gao, S ., Williams, D. F ., Hedrick, J. L . & Yang, Y. Y . The effect of kinetic stability on biodistribution and anti-tumor efficacy of drug-loaded biodegradable polymeric micelles. Biomaterials 34, 3132–3140 (2013).
-
Nederberg, F ., Appel, Due east ., Tan, J. P. K ., Kim, Southward. H ., Fukushima, Chiliad ., Sly, J ., Miller, R. D ., Waymouth, R. M ., Yang, Y. Y . & Hedrick, J. L . A simple approach to stabilized micelles employing mikto-arm terpolymers and stereocomplexes with application in paclitaxel delivery. Biomacromolecules 10, 1460–1468 (2009).
-
Geihe, East. I ., Cooley, C. B ., Simon, J. R ., Kiesewetter, 1000. K ., Edward, J. A ., Hickerson, R. P ., Kaspar, R. L ., Hedrick, J. Fifty ., Waymouth, R. M . & Wender, P. A . Designed guanidinium-rich amphipathic oligocarbonate molecular transporters complex, evangelize and release siRNA in cells. Proc. Natl Acad. Sci. The states 109, 13171–13176 (2012).
-
Engler, A. C ., Wiradharma, N ., Ong, Z. Y ., Coady, D. J ., Hedrick, J. L . & Yang, Y. Y . Emerging trends in macromolecular antimicrobials to fight multi-drug-resistant infections. Nano Today 7, 201—222 (2012).
-
Fukushima, M ., Liu, Due south ., Wu, H ., Engler, A. C ., Coady, D. J ., Maune, H ., Pitera, J ., Nelson, A ., Wiradharma, N ., Venkataraman, S ., Huang, Y ., Fan, W ., Ying, J. Y ., Yang, Y. Y . & Hedrick, J. L . Supramolecular high-aspect ratio assemblies with strong antifungal activity. Nat. Commun. 4, 2861 (2013).
-
Silva, A. D ., Sundberg, Fifty. K ., Ito, H ., Sooriyakumaran, R ., Allen, R. D . & Ober, C. K . A fundamental written report on dissolution beliefs of loftier-resolution molecular glass photoresists. Chem. Mater. 20, 7292–7300 (2008).
-
Tsuruta, T . Contemporary topics in polymeric materials for biomedical applications. Adv. Polym. Sci. 126, 1–51 (1996).
-
Newcomb, C. J ., Sur, S ., Ortony, J. H ., Lee, O.-Due south ., Matson, J. B ., Boekhoven, J ., Yu, J.-M ., Schatz, G. C . & Stupp, Due south. I . Cell death versus prison cell survival instructed past supramolecular cohesion of nanostructures. Nat. Commun. 5, 3321 (2014).
-
Fukushima, K . & Tanaka, M . Biocompatible and biodegradable polymers. Patent application pending JP2014 097237.
-
Hatakeyma, T ., Kasuga, H ., Tanaka, M . & Hatakeyama, H . Cold crystallization of poly(ethylene glcyol)-h2o sytems. Thermochim. Acta 465, 59–66 (2007).
-
Hatakeyma, T ., Tanaka, M . & Hatakeyama, H . Thermal properties of freezing spring water restrained by polysaccharides. J. Biomat. Sci. Polym. Ed. 21, 1865–1880 (2010).
-
Hatakeyma, T ., Tanaka, G . & Hatakeyama, H . Studies on bound h2o restrained past poly(ii-methacryloyloxyethyl phosphorylcholine) (PMPC): comparison of the polysaccharides-water systems. Acta Biomater. half-dozen, 2077–2082 (2010).
-
Hatakeyma, T ., Kishi, A . & Tanaka, M . Comparison of measurement techniques for the identification of bound water restrained by polymers. Thermochim. Acta 532, 159–163 (2012).
-
Hayashi, T ., Tanaka, Y ., Koide, Y ., Tanaka, M . & Hara, Chiliad . Mechanism underlying bioinertness of self-assembled monolayers of oligo(ethyleneglycol)-terminated alkanethiols on aureate: protein adsorption, platelet adhesion, and surface forces. Phys. Chem. Chem. Phys. fourteen, 10194–10206 (2012).
-
Hirata, T ., Matsuno, H ., Tanaka, M . & Tanaka, Yard . Surface segregation of poly(ii-methoxyethyl acrylate) in a mixture with poly(methyl methacrylate). Phys. Chem. Chem. Phys. thirteen, 4928–4934 (2011).
-
Morita, S ., Tanaka, 1000 ., Kitagawa, Chiliad . & Ozaki, Y . Hydration structure of poly(two-methoxyethyl acrylate): comparison with a model monomer. J. Biomat. Sci. Polym. Ed. 21, 1925–1935 (2010).
-
Mochizuki, A ., Kishi, A . & Tanaka, M . Comparative study on water structures in polyHEMA and polyMEA by XRD-DSC simultaneous measurement. J. Appl. Poly. Sci. 111, 476–481 (2009).
-
Tanabe, A ., Morita, South ., Tanaka, M ., Noda, I . & Ozak, Y . Multivariate curve resolution analysis on multi-component water sorption procedure into a poly(two-methoxyethyl acrylate) moving picture. Appl. Spec. 62, 46–50 (2008).
-
Kitano, H ., Nagaoka, K ., Tada, S ., Gemmei-Ide, Thousand . & Tanaka, M . Structure of water incorporated in amphoteric polymer thin films equally revealed by FT-IR spectroscopy. Macromol. Biosci. 8, 77–85 (2008).
-
Morita, S ., Tanaka, M ., Noda, I . & Ozaki, Y . Phase angle description of perturbation correlation analysis and its application to fourth dimension-resolved IR spectra. Appl. Spec. 61, 867–872 (2007).
-
Kitano, H ., Ichikawa, K ., Fukuda, M ., Mochizuki, A . & Tanaka, Yard . The structure of water sorbed to poly(methyl methacrylate) film as examined by FT-IR spectroscopy. J. Colloid Surface Sci. 242, 133–140 (2001).
-
Ichikawa, K ., Mori, T ., Kitano, H ., Fukuda, M ., Mochizuki, A . & Tanaka, G . A FT-IR study on the sorption of water to various kinds of polymer sparse films. J. Polym. Sci. B Polym. Phys. 39, 2175–2182 (2001).
-
Tanaka, Thousand . Design of novel 2D and 3D bio-interfaces using cocky-organization to control cell behavior. Biochim. Biophys. Acta 1810, 251–258 (2011).
-
Oda, Y ., Horinouchi, A ., Kawaguchi, D ., Matsuno, H ., Kanaoka, S ., Aoshima, S . & Tanaka., One thousand . Effect of side-chain carbonyl groups on the interface of vinyl polymers with water. Langmuir thirty, 1215–1219 (2014).
-
Morita, Due south . & Tanaka, M . Effect of sodium chloride on hydration structures of PMEA and p(MPC-r-BMA). Langmuir 30, 10698–10703 (2014).
Acknowledgements
This work is supported past Grants-in-Help and Special Coordination Funds for Promoting Scientific discipline and Engineering of Ministry building of Teaching, Civilisation, Sports, Science and Technology, Japan (KAKENHI). Nosotros greatly acknowledge the financial support from Funding Program for Adjacent Generation Earth-Leading Researchers (NEXT Plan, Nihon).
Author data
Affiliations
Corresponding writer
Rights and permissions
About this article
Cite this commodity
Tanaka, K., Sato, K., Kitakami, E. et al. Blueprint of biocompatible and biodegradable polymers based on intermediate water concept. Polym J 47, 114–121 (2015). https://doi.org/10.1038/pj.2014.129
-
Received:
-
Revised:
-
Accepted:
-
Published:
-
Result Date:
-
DOI : https://doi.org/x.1038/pj.2014.129
Further reading
batchelorfeembirl.blogspot.com
Source: https://www.nature.com/articles/pj2014129
0 Response to "Biodegradable and Biocompatible Polymers for Tissue Engineering Application a Review"
Post a Comment